
For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process. New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). Nov 03, 2019 · approval: New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). If companies follow the steps to approval outlined by fda, then review and approval times will be reduced, and more generic drug products will be available for patients. For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process. 10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. 10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. Administrative and limited scientific review by fda staff to determine completeness. Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda.
10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda. Administrative and limited scientific review by fda staff to determine completeness. New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). Nov 03, 2019 · approval: 10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. If companies follow the steps to approval outlined by fda, then review and approval times will be reduced, and more generic drug products will be available for patients. For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process.
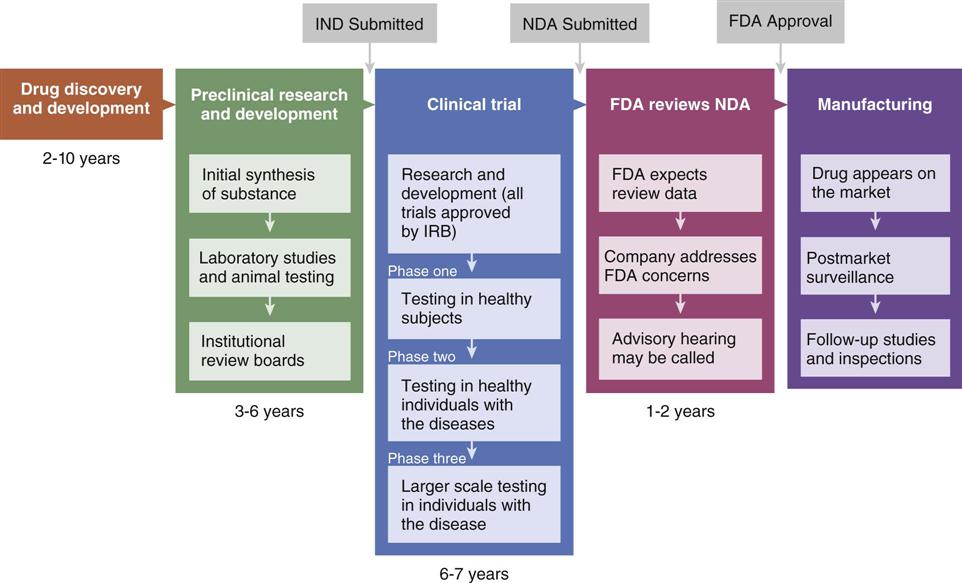
Nov 03, 2019 · approval:
If companies follow the steps to approval outlined by fda, then review and approval times will be reduced, and more generic drug products will be available for patients. Administrative and limited scientific review by fda staff to determine completeness. 10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process. Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda. 10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). Nov 03, 2019 · approval:
Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda. 10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. Administrative and limited scientific review by fda staff to determine completeness. If companies follow the steps to approval outlined by fda, then review and approval times will be reduced, and more generic drug products will be available for patients. For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process. New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). Nov 03, 2019 · approval: 10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda).
For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process.
10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. Nov 03, 2019 · approval: New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). If companies follow the steps to approval outlined by fda, then review and approval times will be reduced, and more generic drug products will be available for patients. Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda. For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process. Administrative and limited scientific review by fda staff to determine completeness. 10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda).
Administrative and limited scientific review by fda staff to determine completeness. New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process. New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). 10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. If companies follow the steps to approval outlined by fda, then review and approval times will be reduced, and more generic drug products will be available for patients. Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda.

Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda.
If companies follow the steps to approval outlined by fda, then review and approval times will be reduced, and more generic drug products will be available for patients. Nov 03, 2019 · approval: Administrative and limited scientific review by fda staff to determine completeness. New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda). Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda. For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process. 10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved. 10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved.
Nov 03, 2019 · approval: fda approval process. Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda.

If companies follow the steps to approval outlined by fda, then review and approval times will be reduced, and more generic drug products will be available for patients.

Nov 03, 2019 · approval:
/https:%2F%2Fblogs-images.forbes.com%2Fdavidkroll%2Ffiles%2F2017%2F03%2FCaliff-CED-02.28.17.jpg)
New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda).

For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process.

10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved.

10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved.

If companies follow the steps to approval outlined by fda, then review and approval times will be reduced, and more generic drug products will be available for patients.

Administrative and limited scientific review by fda staff to determine completeness.

Administrative and limited scientific review by fda staff to determine completeness.

Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda.

Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda.

Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda.

Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda.
_Typical_Drug_Development_and_Approval_Process_(35856478702).jpg)
If companies follow the steps to approval outlined by fda, then review and approval times will be reduced, and more generic drug products will be available for patients.

Nov 03, 2019 · approval:

Nov 03, 2019 · approval:

Nov 03, 2019 · approval:

Nov 03, 2019 · approval:

Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda.

Nov 03, 2019 · approval:

10 critics, however, argue that the fda standards are not sufficiently rigorous, allowing unsafe or ineffective drugs to be approved.

Nov 03, 2019 · approval:

Administrative and limited scientific review by fda staff to determine completeness.

New drugs receive extensive scrutiny before fda approval in a process called a new drug application (nda).

Nov 03, 2019 · approval:

Following a successful submission of a premarket approval (pma) or a humanitarian device exemption (hde), the device is given approval by fda.

For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process.

For class iii medical devices to be legally marketed they must undergo a rigorous review and approval process.

Administrative and limited scientific review by fda staff to determine completeness.
Posting Komentar untuk "Fda Drug Approval Process Steps / FDA Drug Approval Process"